Researchers use precise light control to reversibly switch left and right-handed helices, expanding possibilities for advanced optical and electronic materials
In a striking demonstration of molecular control, a team of Japanese scientists has harnessed light to reverse the twist in self-assembling molecules. The study led by Professor Shiki Yagai from Chiba University identifies how trace residual aggregates in photo-responsive azobenzene solutions can reverse helical chirality through secondary nucleation. By using precise control of ultraviolet and visible light, the researchers could switch between the rotation of helices, offering a breakthrough for novel materials with tunable properties.
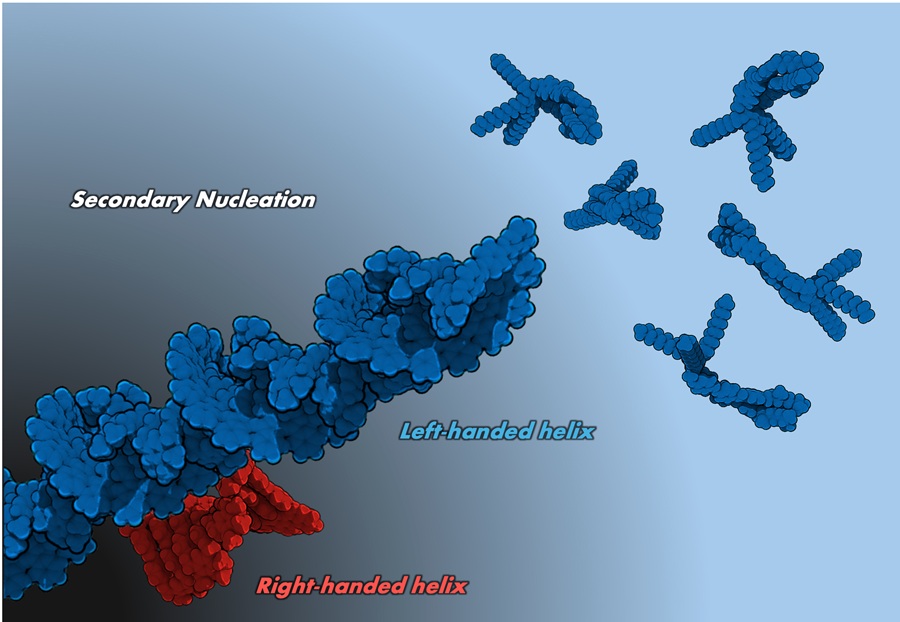
Image title: Influence of residual aggregates in photoresponsive solutions
Image caption: Researchers identified how the presence of residual aggregates can influence the supramolecular chirality, opening new avenues in electronic materials.
Image credit: Takuho Saito from Chiba University, Japan
Image license: Original content
Usage restrictions: Credit must be given to the creator.
Self-assembly or self-organization in molecular science refers to the phenomena where molecules spontaneously gather and form ordered structures, a unique property of materials used to develop optical and electronic materials. In a step towards fine-tuning this property, researchers from Japan successfully elucidated a technique where a small amount of residual aggregates drastically altered the self-assembly process of photo-responsive molecules. The research team was led by Professor Shiki Yagai from the Graduate School of Engineering, Chiba University, including Assistant Professor Takuho Saito from Nagoya University (at the time of research), Mr. Daisuke Inuoe, and Assistant Professor Yuichi Kitamoto from Tohoku University, as major contributors of this work. The findings of their study were published online in Nature Nanotechnology on April 11, 2025.
In recent years, there has been an increasing focus in research on controlling the size and hierarchical structures of self-assembled aggregates, which could help achieve aggregates with desired properties. However, self-assembly is a dynamic process and requires precise control. “During the process of self-assembly, the molecules repeatedly continue to associate and dissociate,” explains Prof. Yagai, “Even minute impurities or slight changes in the conditions can impact the final structure of the formed aggregates.”
For the study, the research team focused on the self-assembly of a chiral, photoresponsive azobenzene that naturally forms left-handed helical aggregates. The team discovered that the presence of a small amount of residual aggregates within the solution induces a drastic change in the assembly process and leads to the formation of right-handed helical aggregates instead. Moreover, being photoresponsive, controlling the exposure to light also modifies the timing of molecular assembly. Using precise control of these two properties together, the researchers successfully manipulated the formation of either left-handed or right-handed helical aggregates as required.
In spectroscopic and molecular modeling studies, the team found that when the scissor-shaped azobenzene molecule is dissolved in an organic solvent at room temperature, it forms a closed scissor-like folded structure that further extends into a helical assembly. Prof. Yagai explains the formation of left-handed assembly, saying, “The molecule contains a carbon atom that has four different atomic groups and therefore exhibits chirality. These molecules fold like left-handed scissors and twist to form a left-handed helical stacking of the assembly.”
Since these are photoresponsive molecules, when the stacked helical structures are exposed to weak ultraviolet (UV) light, the helical assembly disassembles back into individual molecules, and upon subsequent exposure to visible light, the molecules reassemble into helical structures again. Interestingly, under certain conditions, the resulting helical aggregates were found to be right-handed instead of left-handed, and exposure to stronger UV light followed by visible light led to the regeneration of the original left-handed helical aggregates.
By closely investigating this mechanism, the team found that when solutions were exposed to weak UV light, there was a minute amount of residual left-handed helical aggregates that remained unchanged, and these aggregates acted as nucleation sites forming oppositely directed helical assemblies. “This remarkable phenomenon is called ‘secondary nucleation,’ which explains why meta-stable right-handed aggregates are preferably formed instead of left-handed aggregates,” says Prof. Yagai.
In addition to this, the team also discovered the role of light intensity in the molecular assembly process. Prof. Yagai explains, “We identified that the intensity of visible light potentially affected the timing of the assembly. Strong visible light promoted rapid assembly while minimizing the influence of the residual aggregates. In contrast, weaker intensity magnifies the effect of the residual aggregates.”
Therefore, by optimizing the intensities of UV and visible light, the researchers successfully controlled the switching between left- and right-handed helical structures, which were dependent on the influence of the residual aggregates. Moreover, it was also found that the stable left-handed aggregates and meta-stable right-handed aggregates also exhibit opposite electron spin polarization, which signifies the tuning of electronic characteristics of the helices.
Overall, this study aimed to explore the critical role of residual aggregates and explained how light-enabled fine-tuning can result in the fabrication of novel functional materials, giving promising insights into the field of material science.
About Professor Shiki Yagai from Chiba University
Professor Shiki Yagai is a well-established researcher at Chiba University, Japan. He currently serves as a professor at the Graduate School of Engineering, Chiba University, Japan. He has authored over 200 publications, contributing to the fields of molecular self-assembly, supramolecular polymers, and functional dyes with a focus on their nanostructural control. Prof. Yagai leads the Grant-in-Aid for Transformative Research Areas (A) project titled “Materials Science of Meso-Hierarchy” which spans from 2023 to 2027. This project focuses on exploring the hierarchical structures of materials to develop innovative functional materials at the mesoscopic scale. The initiative aims to deepen the understanding of meso-hierarchical structures and their impact on material properties, potentially leading to breakthroughs in material design and applications.
See more at https://mesohierarchy.jp/en/
Funding:
This research was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (JP20K21216, JP21J20988, JP23H04873), Mazda Foundation Research Grant and Large Synchrotron Radiation Facility SPring-8 Experiment Project (proposal number 20160027).
Reference:
Title of original paper: Inversion of supramolecular chirality by photo-enhanced secondary nucleation
Authors: Takuho Saito¹, Daisuke Inoue², Yuichi Kitamoto³, Hiroki Hanayama², Takatoshi Fujita⁵, Yuki Watanabe⁶, Masayuki Suda⁶,⁷, Takashi Hirose⁸, Takashi Kajitani⁹,¹⁰, and Shiki Yagai²,⁴
Affiliations:
¹Division of Advanced Science and Engineering, Graduate School of Science and Engineering, Chiba University
²Department of Applied Chemistry and Biotechnology, Graduate School of Engineering, Chiba University
³Department of Biomolecular Engineering, Graduate School of Engineering, Tohoku University
⁴Institute for Advanced Academic Research, Chiba University
⁵Institute for Quantum Life Science, National Institutes for Quantum Science and Technology
⁶Department of Molecular Engineering, Graduate School of Engineering, Kyoto University
⁷JST-FOREST Program
⁸Institute for Chemical Research, Kyoto University
⁹Open Facility Development Office, Open Facility Center, Institute of Science Tokyo
¹⁰RIKEN SPring-8 Center
Journal: Nature Nanotechnology
DOI: 10.1038/s41565-025-01882-8
Contact: Shiki Yagai
Professor, Graduate School of Engineering, Chiba University
Email: yagai@faculty.chiba-u.jp
Public Relations Office, Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: koho-press@chiba-u.jp
Tel: +81-43-290-2018
Recommend
-

An insect and machine enthusiast delves into the flight mechanism of living organisms: From the mosquito flapping to the high-performance drones
2024.03.12
-

The Science Behind Nature’s Touch: Horticultural Therapy and Community Well-Being
2025.01.14
-

The Global Goal of Carbon Neutrality by 2050 (Part 2): A decarbonized society from a local perspective
2023.07.14


