A new enzyme-based molecular design can lower the retention of radioactive agents in the kidneys during radiotheranostic applications
Radiotheranostics is an approach that involves the use of a single radioactive agent for disease diagnosis and treatment. However, the use of low-molecular-weight proteins and peptides can lead to unwanted accumulation of the associated radioactive metabolites in the kidneys, which interferes with diagnostic accuracy and may cause side effects. Researchers from Japan recently overcame this limitation by developing novel radiolabeled antibody fragments capable of enhancing overall treatment outcomes and imaging accuracy.
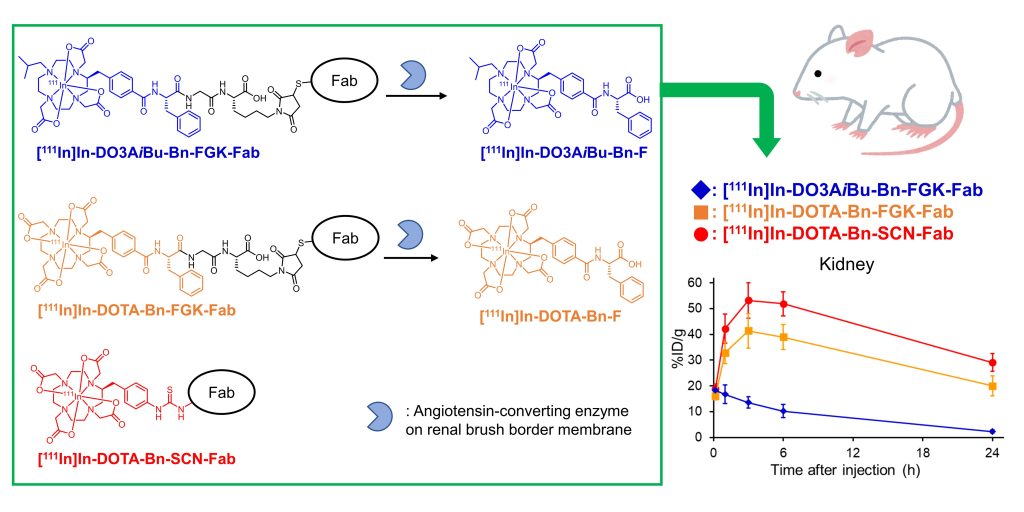
Image Title: A new enzyme-based molecular design with key implications in radiotheranostics
Image Caption: 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-based
reagents, when conjugated to antibody fragments and injected into mice, produced the metabolites [111In]In-DO3AiBu-Bn-F and [111In]In-DOTA-Bn-F, which were dissimilarly eliminated by the kidneys. Quite notably, [111In]In-DO3AiBu-Bn-FGK-Fab showed the lowest renal localization without impairing tumor accumulation, thus paving the way for the possible clinical evaluation of this radiotheranostic approach.
Image Credit: Suzuki et al., Chiba University
Image Source Link: https://pubs.acs.org/doi/10.1021/acs.jmedchem.3c00258
License Type: CC BY-NC-ND 4.0
Radiotheranostics embodies the convergence of diagnostic and therapeutic radiopharmaceuticals into a unified platform. In cancer treatment, radiotheranostic procedures typically involve the use of antibodies that bind to proteins abundantly found on the surface of cancerous cells. The antibodies are labeled with a suitable radioisotope, which facilitates imaging procedures used to diagnose cancer and can be used to target cancerous cells and bombard them with deadly radiation as a form of treatment.
Although radiolabeled antibodies show promise as a treatment for cancer, several hurdles impede their clinical translation. The most notable impediment is slow blood clearance, which causes bone marrow toxicities. Meanwhile, radiolabeled antibody fragments show rapid blood clearance, but cause accumulation and slow clearance of the radiolabeled particles (or radiometabolites) from the kidneys, also known as renal retention. The challenge of elevated radioactivity in the kidneys from the radiolabeled antibody fragments—detrimentally affecting both diagnosis precision and therapy outcomes—has hindered the progress of radiotheranostics applications for more than three decades.
Recently, medicinal chemistry researchers from Japan’s Chiba University developed new radiolabeled antibody fragments and tested them in a murine model. They found that the novel radioisotope-labeled antibody fragments facilitate the rapid elimination of the resulting radiometabolites from murine kidneys. More specifically, the team developed 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-based reagents for radiotheranostic applications. Their study was made available online on June 16, 2023, and published in Volume 66, Issue 13 of the journal Medicinal Chemistry on July 13, 2023.
When asked about their novel molecular design, Dr. Hiroyuki Suzuki, the lead author of the study from the Graduate School of Pharmaceutical Sciences, Chiba University, explains, “We have developed a radiolabeled antibody fragment that reduced renal radioactivity levels, where we used DOTA as the radiolabeling part. DOTA is a promising chelator, where a chelator is a molecule that forms a stable complex with a metal ion, such as a radiometal; this would find potential radiotheranostic applications.” The team also developed an additional DOTA derivative and tested its efficacy using a murine model.
Prior research published by the same group addressed the issue of renal retention by devising a “renal brush border” strategy. It involved the introduction of an enzyme-cleavable linkage—a chemical bond that can be broken only by certain enzymes—between the radiolabel and the conjugation moiety that links it to the antibody. The enzymes on the renal released brush border membrane recognize and cleave this linkage, thus allowing the rapid elimination of radiometabolites through urine.
Building upon the renal brush border strategy, Dr. Suzuki’s team developed new radioiodinated antibody fragments with enzyme-cleavable linkages. After injecting the newly developed DOTA-based reagents into tumor-bearing mice, the team performed physiological imaging using single-photon emission computed tomography, which detects the gamma radiation emitted by the injected radiopharmaceuticals.
Experiments conducted on laboratory mice showed rapid renal excretion of the generated radiometabolites. Quite notably, one of the radiolabeled antibody fragments ([111In]In-DO3AiBu-Bn-FGK-Fab) showed substantial accumulation in the murine tumors, which highlights their potential as an effective diagnostic tool, paving the way for future clinical evaluation. Although the study authors admit the requirement of additional efforts to confirm its applicability to therapy, their findings show promising clinical applications.
Therefore, this research could accelerate the progress of radiotheranostics, combining improved diagnostic precision and therapy while minimizing potential side effects. “This study will contribute to the development of radiopharmaceuticals that can provide radiotheranostics capable of more accurate diagnosis and therapy with reduced side effects. Radiotheranostics can contribute to personalized medical approaches with tremendous benefits to the treated patients,” concludes Dr. Hiroyuki Suzuki.
About Assistant Professor Hiroyuki Suzuki
Dr. Hiroyuki Suzuki is an Assistant Professor at the Graduate School of Pharmaceutical Sciences, Chiba University. Dr. Suzuki’s research interests include diverse aspects of radiochemistry, radiopharmaceutical synthesis, imaging techniques, and therapeutic applications, contributing to advancements in nuclear medicine and cancer therapy. He has authored around 27 scientific publications.
Reference:
Title of original paper: Reduction of the Renal Radioactivity of 111In-DOTA-Labeled Antibody Fragments with a Linkage Cleaved by the Renal Brush Border Membrane Enzymes
Authors: Hiroyuki Suzuki, Mari Araki, Kouki Tatsugi, Kento Ichinohe, Tomoya Uehara, and Yasushi Arano
Affiliation:
Laboratory of Molecular Imaging and Radiotherapy, Graduate School of Pharmaceutical Sciences, Chiba University, Chiba 260-8675, Japan
Journal: Journal of Medicinal Chemistry
DOI: 10.1021/acs.jmedchem.3c00258
Contact:
Hiroyuki SUZUKI
Graduate School of Pharmaceutical Sciences, Chiba University
Email: h.suzuki@chiba-u.jp
Public Relations Office, Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: koho-press@chiba-u.jp
Tel: +81-43-290-2018





