Researchers shed light on the carbonation of cement-based materials, essential for developing advanced carbon dioxide-absorbing building materials
Cement-based materials provide a potential solution for mitigating climate change by trapping and storing atmospheric carbon dioxide as minerals, via a process known as carbonation. Despite extensive studies, however, the exact mechanism of this process is not yet understood. Now, researchers have conducted a comprehensive investigation of carbonation reaction using a new method, revealing the role of structural changes and water transport, paving the way for advanced carbon dioxide-absorbing building materials.
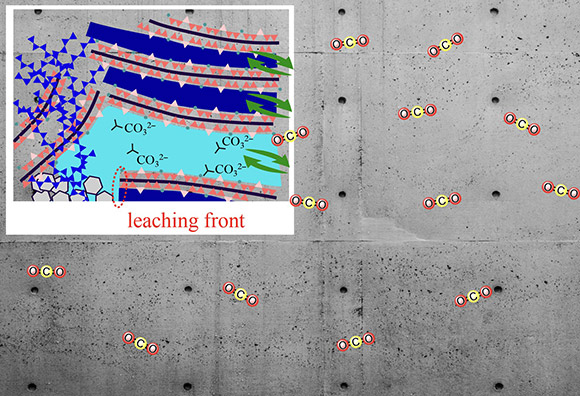
Image title: Relationship between the carbonation of cementitious materials and water transport phenomena
Image caption: The interplay of water transport and related structural changes in cement-based materials help absorb atmospheric carbon dioxide in the form of CO32-.
Image credit: Takahiro Ohkubo from Chiba University
Image license: Original Content
Usage restrictions: Cannot be reused without permission
Carbon dioxide (CO2) emissions are currently one of the leading causes of global warming. Cement-based materials have shown promising applications in capturing and solidifying CO2 as minerals through a process called carbonation, offering a potential solution to mitigate the challenges associated with climate change. Consequently, numerous studies have been conducted on the carbonation of cement-based materials to improve the efficiency of carbonation.
Put simply, carbonation in cement paste involves the dissolution of CO2 in water, followed by interaction with calcium silicate hydrates (C–S–H), formed during the hydration of raw materials. During this reaction, the dissolved CO2 forms carbonate ions (CO32-), and further reacts with calcium ions (Ca2+) from C–S–H to create calcium carbonate precipitates. However, despite extensive studies with varying parameters, the complete explanation of carbonation mechanisms is not clearly understood due to the unstable nature of cement paste compounds.
Previous studies have shown that carbonation is strongly impacted by relative humidity (RH), CO2 solubility, calcium/silicate (Ca/Si) ratio, and concentration and saturation level of water in C–S–H. Moreover, it is also important to understand the influence of ions and water transport through the nanometer-sized pores in C–S–H layers, known as gel-pore water.
To answer these questions, Associate Professor Takahiro Ohkubo from the Graduate School of Engineering at Chiba University with his team of researchers, including Taiki Uno from Chiba University, Professor Ippei Maruyama and Naohiko Saeki from The University of Tokyo, Associate Professor Yuya Suda from University of Ryukyus, Atsushi Teramoto from Hiroshima University, and Professor Ryoma Kitagaki from Hokkaido University investigated the mechanism of carbonation reaction under different Ca/Si ratios and RH conditions. Their study was published in The Journal of Physical Chemistry C on July 08, 2024. “The role of water transport and carbonation-related structural changes remains an open question. In this study we used a new method to study these factors, using 29Si nuclear magnetic resonance (NMR) and 1H NMR relaxometry, which has been established as an ideal tool for studying water transport in C–S–H,” says Associate Professor Ohkubo.
To study the carbonation process, the researchers synthesized C–S–H and subjected them to accelerated carbonation using 100% CO2, far higher than atmospheric levels. “Natural carbonation in cement materials occurs over several decades by absorbing atmospheric CO2, making it difficult to study in a lab setting. Accelerated carbonation experiments with elevated CO2 concentrations provide a practical solution to this challenge,” explains Associate Professor Ohkubo. The samples were synthesized under varying RH conditions and Ca/Si ratios. Further, they studied the C–S–H samples using 29Si NMR, and the water exchange processes using 1H NMR relaxometry under a deuterium dioxide (D2O) atmosphere.
The researchers found that the structural changes induced by the carbonation reaction, including the collapse of the C–S–H chain structure and changes in the pore size were heavily influenced by the Ca/Si ratio of the C–S–H chain and RH conditions. Additionally, lower RH conditions and a high Ca/Si ratio resulted in smaller-sized pores, suppressing the leaching of Ca2+ ions and water from the interlayer space to gel-pores, leading to inefficient carbonation. “Our study shows that the carbonation process occurs due to a combination of structural modifications and mass transfer, signifying the importance of studying their interplay, rather than just structural changes,” says Associate Professor Ohkubo.
Further highlighting the implications of the present study, Associate Professor Ohkubo adds, “Our findings can contribute to developing new building materials that can absorb large amounts of atmospheric CO2. Additionally, carbonation reactions are also common in organic matter and hence, our new approach will also help to understand the carbonation of compounds in the natural environment.”
In conclusion, this study sheds light on the carbonation reaction of cement-based materials, offering a potential solution to CO2 reduction.
About Associate Professor Takahiro Ohkubo
Takahiro Ohkubo is currently an Associate Professor at the Graduate School of Engineering at Chiba University, Japan since 2015. He received his M.S. degree from Chiba University in 2000 and his Ph.D. degree from Tokyo Institute of Technology in 2005. Before joining Chiba University, he worked at the Japan Atomic Energy Agency and the National Institute of Advanced Industrial Science and Technology. His research interests include inorganic chemistry, computational science, and nuclear magnetic resonance. He has published around 60 research articles, which have been cited over 500 times.
Funding information:
This study was based on the results obtained from a project (JPNP21023) commissioned by the New Energy and Industrial Technology Development Organization (NEDO). A part of this work was supported by JSPS KAKENHI (Grant Nos. 23H00203 and 23H04096).
Reference:
Title of original paper: Understanding the Carbonation Phenomenon of C–S–H through Layer Structure Changes and Water Exchange
Authors: Taiki Uno1, Naohiko Saeki2, Ippei Maruyama2, Yuya Suda3, Atsushi Teramoto4, Ryoma Kitagaki5, and Takahiro Ohkubo1
Affiliations:
1Graduate School of Engineering and Science, Chiba University
2Graduate School of Engineering, The University of Tokyo
3Faculty of Engineering, University of the Ryukyus
4Graduate School of Advanced Science and Engineering, Hiroshima University
5Graduate School of Engineering, Hokkaido University
Journal: The Journal of Physical Chemistry C
DOI: 10.1021/acs.jpcc.4c01714
Contact: Takahiro Ohkubo
Graduate School of Engineering, Chiba University
Email: ohkubo.takahiro@faculty.chiba-u.jp
Public Relations Office, Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: koho-press@chiba-u.jp
Tel: +81-43-290-2018





