Scientists now develop sodium carbonate-activated polyacrylonitrile-based carbon fibers for nitrate ion removal from natural groundwater sources
Nitrate ions released into the water bodies due to human activities can have adverse effects on aquatic and terrestrial ecosystems. Conventional adsorption materials that are used for treating water contamination are incapable of removing nitrate. To deal with this issue, researchers from Chiba University, Japan, produced new polyacrylonitrile-based carbon fibers that, when activated with sodium carbonate and heat-treated, can exhibit excellent nitrate adsorption properties.
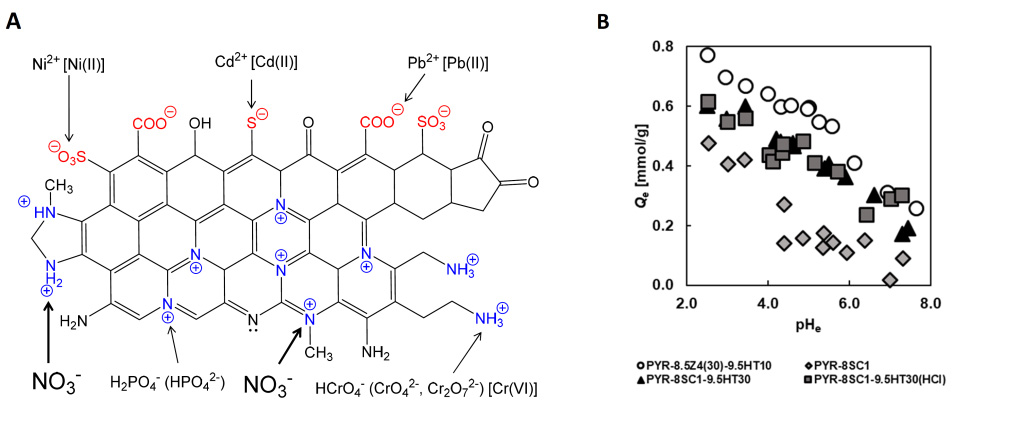
Title: Schematic diagram of surface functional groups on the carbonaceous adsorbent developed by the researchers.
Image caption: Researchers at Chiba University, Japan, prepared polyacrylonitrile (PAN)-based activated carbon fibers that are capable of removing nitrate ions from the environment. A. Adsorbent structure and its surface functional groups. Quaternary nitrogen (N-Q), including graphitic nitrogen, and aliphatic amine could be effective for the removal of anionic pollutants, such as nitrate, phosphate, chromium, and arsenic anions. B. A graph representing the influence of equilibrium solution pH (pHe) on the amount of NO3– adsorption.
Image credit: Motoi Machida from Chiba University, Japan
License type: Original Content
Usage restrictions: Cannot be reused without attribution
Nitrate ion is a common pollutant produced by municipal waste treatment systems, agricultural run-offs, and livestock waste. Although an essential component of fertilizers and necessary for growing food, nitrates can be harmful when left to circulate in our ecosystem without proper treatment. It can, for instance, lead to algal bloom in water bodies, which reduces the amount of dissolved oxygen in water, posing a threat to aquatic organisms. Nitrate ions have also been associated with blood disorders in infants and digestive cancers in adults. Hence, an easy and cost-effective way to remove nitrate ions from our ecosystem is crucial.
Over the years, researchers have tried and tested several materials that can remove nitrate ions via adsorption. A popular approach is to use adsorbent materials, such as activated carbon fibers (ACF). The porous structure of ACFs enables the introduction of a wide range of functional groups that can be chosen per the adsorption requirements. They are also easy to recover and reuse. Unfortunately, there are certain shortcomings of ACF that hinder its applications.
Prof. Motoi Machida from Chiba University, Japan, who leads the research on nitrate ion removal, sheds some light on the issues faced. “Carbonaceous materials like activated carbons are usually functionalized with negatively charged functional groups, such as carboxy, carbonyl or phenol, which show excellent adsorption performance for positively charged ions and organic pollutants but are ineffective against inorganic negatively charged ions,” he explains. “So, we wanted to develop a durable ACF-based material that can efficiently adsorb nitrate ions and retain adsorption capacity for multiple uses.”
In a recent breakthrough made available online on 10 October 2022 and published in SN Applied Sciences on 22 October 2022, Prof. Machida, along with Ms. Natsuho Sato and Associate Prof. Yoshimasa Amano from Chiba University, designed sodium carbonate activated, polyacrylonitrile (PAN)-based ACF with high nitrogen content for nitrate ion removal. To increase the adsorption property of the PAN-ACF, the team activated them with sodium carbonate at 800°C. They further heat-treated the fibers at 950°C, which reduced the nitrogen content but increased the amount of quaternary nitrogen (N-Q), a positively charged functional group capable of nitrate adsorption.
Following the preparation process, the team carried out tests to analyze the nitrate ion adsorption dynamics of the material. The results showed that heat treatment increased the nitrate adsorption capacity by eliminating nitrate-repelling functional groups, such as –COOH and –COO–. The PAN-ACF material showed excellent adsorption properties, with an adsorption capacity of 0.5 mmol/g nitrate ions from water.
The team also conducted long-term degradation studies under different storage conditions to figure out the best way of storing the material for optimum reuse results. They found that keeping the ACFs in hydrochloric acid solution or under vacuum kept them stable. The adsorption capacity and degradation studies revealed that the material showed a consistent adsorption rate at a pH of 4-5, an encouraging find since 4-5 is the pH range of groundwater.
“Our new robust material can withstand treatments with boiling water, and strong acidic and basic solutions, thereby still easily regenerate with minimal loss in adsorption capacity,” adds Prof. Machida.
Overall, the new PAN-ACF presented in the study can not only treat environmental water by removing harmful nitrate ions but also help reduce the dependence on conventional plastic material-based ion exchange resins by replacing them with more environment-friendly active carbon-based ion removers.
About Professor Motoi Machida
Dr. Motoi Machida is a Professor at the Division of Applied Chemistry and Biotechnology at Chiba University, Japan. He received his bachelor’s degree from Hokkaido University, Sapporo, in 1983, studying the acid-base catalyst. He then started working on hydrotreating catalysts and received his Ph.D. in 1998. He moved to Chiba University as an associate professor in 2001 and started developing adsorbents for water purification. He stayed at Laurentian University, Ontario, working with Dr. Luis Mercier on mesoporous silica in 2011. His research group is currently investigating hetero-atom functionalized adsorbent surface for the purification of contaminated water.
Reference:
Title of original paper: Adsorption characteristics of nitrate ion by sodium carbonate activated PAN‑based activated carbon fiber
Authors: Natsuho Sato1, Yoshimasa Amano2,3 and Motoi Machida2,3
Affiliations:
- Graduate School of Science and Engineering, Chiba University
- Graduate School of Engineering, Chiba University
- Safety and Health Organization, Chiba University
DOI: 10.1007/s42452-022-05191-w
Contact
Motoi MACHIDA
Graduate School of Science and Engineering, Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
TEL: +81 43 290 3559
Email: machida@faculty.chiba-u.jp
Public Relations Office, Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522, JAPAN
Email: koho-press@chiba-u.jp
Recommend
-

Viewing a Diverse World through the Lens of Agriculture and Food: Enriching Global SDGs Education with Insights from Extensive Overseas Field Research
2023.08.31
-

Decoding Epigenome Modification Errors: Exploring Immunological Mechanisms by a Two-way Player in Biology and Data Science
2024.06.27
-

‘Synergistic Campus Evolution with the Community’ Chiba University Design Research Institute (Part 2): Igniting ‘Cross-fertilization’ for a Revolutionary Vision
2023.12.25

