Using a novel co-culturing technique, researchers shed light on the interactions between immune and vascular cells in the context of a rare genetic disease
Patients with Werner syndrome (WS), a rare genetic disease characterized by premature aging, tend to develop atherosclerosis earlier in life. However, the underlying mechanisms are unclear. Now, researchers have developed an innovative co-culturing technique by which the immune cells and vascular cells derived from induced pluripotent stem cells (iPSC) from patients with WS are grown together to analyze their interactions. Understanding the mechanisms underlying WS-associated atherosclerosis can augment the development of potential treatments.
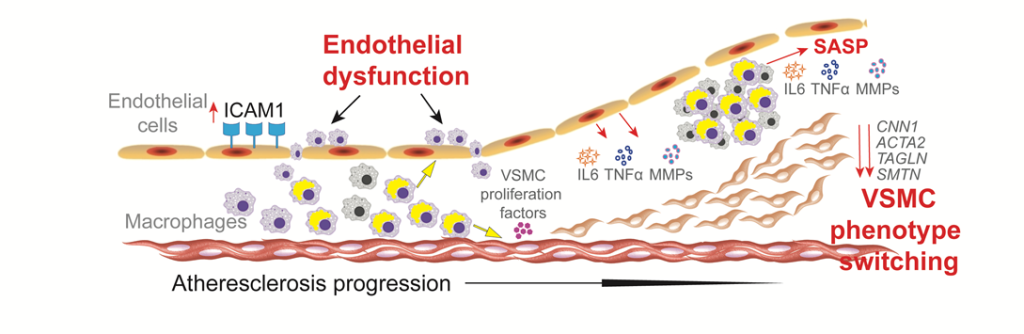
Image title: Atherosclerosis is a common complication in patients with Werner syndrome (WS)
Image caption: Atherosclerosis is a leading cause of death in patients with WS, a rare genetic disease associated with premature aging. Now, a study by researchers from Japan reveals that the buildup of plaque in the arteries, which can eventually cause a stroke or heart attack, is mostly driven by macrophages in an abnormal pro-inflammatory state.
Image credit: Sudip Kumar Paul from Chiba University
Image license: Original content
Restriction: You are free to share and adapt the material. Attribution to the author is required.
Approximately one in every 20,000 to 40,000 children born in Japan and about one in every 100,000 throughout the world bear a mutation in the WRN gene. This gene is responsible for producing the Werner protein, which belongs to the family of human helicases and is responsible for the maintenance of genomic stability, DNA replication, repair of DNA damage, and telomere maintenance. The mutation in the WRN gene causes a rare genetic disease known as Werner syndrome (WS). Since the affected cells cannot repair DNA damage correctly, patients with WS undergo accelerated aging in early adolescence or young adulthood. Consequently, these patients become more susceptible to age-related diseases, including cataracts, diabetes, and osteoporosis, at a younger age.
However, one of the leading causes of premature death among patients with WS is early-onset atherosclerosis, which involves the buildup of plaque inside and on the arteries. Despite many efforts, the precise mechanisms that predispose patients with WS to atherosclerosis at an early age remain unclear. This gap in understanding is generally attributed to the fact that atherosclerosis involves a variety of chemical signaling exchanged between different types of cells.
Now, however, to address this knowledge gap, a research team led by Associate Professor Naoya Takayama from Chiba University, Japan, has recently developed a novel in vitro co-culture system that can accurately represent atherosclerosis, allowing for in-depth molecular analyses. Their latest findings on WS using this system were published in Nature Communications on June 10, 2024.The study was co-authored by Sudip Kumar Paul as the first author along with Koutaro Yokote from Chiba University and Koji Eto, affiliated with Chiba University and Center for iPS Cell Research and Application, Kyoto University.
The developed in vitro co-culture system relies on induced pluripotent stem cells (iPSCs), which have recently garnered significant attention in the medical and life science fields. Simply put, iPSCs are essentially adult cells that were reprogrammed to an embryonic-like state. By stimulating them in specific ways, iPSCs can then transform into almost any desired type of cell and can be cultured accordingly. Since iPSCs can be readily induced from a person’s skin or blood cells, their use allows for detailed studies of how a patient’s genetic makeup affects the way in which specific tissues behave.
By leveraging iPSCs obtained from WS patients and non-WS (healthy) subjects, the researchers differentiated key cells involved in atherosclerosis — macrophages (a type of immune cell), vascular endothelial cells, and vascular smooth muscle cells (components of the blood vessels). Subsequently, they not only analyzed the features of these cells individually and their responses to various conditions, but also analyzed the interplay in co-culture of macrophage–vascular endothelial cells and macrophage–vascular smooth muscle cells together.
Using various analytical techniques such as transcriptomics and open chromatin analysis (which reveal the genes involved in a process), the researchers could explain the occurrence of atherosclerosis in patients with WS, by observing the interactions between the vascular and immune cells. “Our study has revealed that inflammation triggered by mobile genetic elements called retrotransposons and interferon signaling are responsible for atherosclerosis in patients with WS. Macrophages in a pro-inflammatory state are the main malefactors that cause the development of and drive atherosclerosis in these patients, independent of other risk factors,” explains Dr. Takayama.
Interestingly, the researchers could partially restore the correct functioning of the cultured WS cells by silencing interferon signaling in macrophages. As per Dr. Takayama, this could become the basis for effective treatment for patients with WS. Adding further, he says, “The development of inhibitors targeting the interferon signaling pathway holds promise for reducing the incidence of stroke and heart attacks in patients with WS.”
In general, the proposed co-culturing approach could be used to study atherosclerosis efficiently. “We could successfully observe the interactions between immune cells and vascular cells with a uniform genetic background using our new culture technique. Hopefully, it will facilitate the development of effective drugs against atherosclerosis,” concludes Dr. Takayama optimistically.
Further studies on atherosclerosis in patients with WS will be needed to substantiate these findings. However, the efforts spearheaded by Dr. Takayama and his team will undoubtedly help develop efficient management strategies and eventually improve the quality of life of patients with WS.
About Associate Professor Naoya Takayama
Dr. Naoya Takayama is currently an Associate Professor at the Graduate School of Medicine of Chiba University, Japan. His research mainly includes cell biology in relation to stem cells, covering a wide range of medical topics. He has also developed novel analytical methodologies to shed light on the specific mechanisms of pathologies. He has published around 50 peer-reviewed articles in reputable journals. His publications have been cited more than 5,400 times. Dr. Takayama has published in esteemed journals like Cell Stem Cell, Blood, Nature Communications, Journal of Clinical Investigation, Stem Cell Reports, and Journal of Experimental Medicine, to name a few.
Funding:
This research was supported by the Japanese Government MEXT (Monbukagakusho) scholarship, the Tokyo Biochemical Research Foundation, the JSPS KAKENHI (Grant Number: 23K18284), the Japan Agency for Medical Research and Development (Grant Numbers: JP21bm0804016, JP21jm0210096, JP22ym0126066, JP23ej0109622), and the Ministry of Health, Labor and Welfare of Japan (Grant Number: JPMH21FC1016).
Reference:
Title of original paper: Retrotransposons in Werner syndrome-derived macrophages trigger type I interferon-dependent inflammation in an atherosclerosis model
Authors: Sudip Kumar Paul1, Motohiko Oshima2, Ashwini Patil3, Masamitsu Sone1, Hisaya Kato4, Yoshiro Maezawa4, Hiyori Kaneko4, Masaki Fukuyo5, Bahityar Rahmutulla5, Yasuo Ouchi1,6, Kyoko Tsujimura1, Mahito Nakanishi7, Atsushi Kaneda5, Atsushi Iwama2, Koutaro Yokote4, Koji Eto1,8, and Naoya Takayama1
Affiliations:
- Department of Regenerative Medicine, Graduate School of Medicine, Chiba University
- Division of Stem Cell and Molecular Medicine, Center for Stem Cell Biology and Regenerative Medicine, The Institute of Medical Science, The University of Tokyo
- Combinatics Inc.
- Department of Endocrinology, Hematology and Gerontology, Graduate School of Medicine, Chiba University
- Department of Molecular Oncology, Graduate School of Medicine, Chiba University
- Gene Expression Laboratory, Salk Institute for Biological Studies
- TOKIWA-Bio, Inc.
- Department of Clinical Application, Center for iPS Cell Research and Application, Kyoto University
Journal: Nature Communications
DOI: 10.1038/s41467-024-48663-w
Contact: Naoya Takayama
Graduate School of Medicine, Chiba University
Email: tnaoya19760517@gmail.com
Public Relations Office, Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: koho-press@chiba-u.jp
Tel: +81-43-290-2018
International Public Communications Office,
Center for iPS Cell Research and Application (CiRA),
Kyoto University
Address: 53 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507 JAPAN
Email: media@cira.kyoto-u.ac.jp
Tel: +81-75-366-7005





