Researchers reveal auto-antibodies to type I IFNs block IFN signaling, contributing to severe COVID-19 by impairing the immune response
Recently, scientists found that some people who suffered from COVID-19 had auto-antibodies targeting their own type 1 interferons, which are important immune signaling proteins. Now, researchers conducted an in-depth study on 123 Japanese COVID-19 patients to clarify just how common these auto-antibodies are among severe cases and how they affect the immune system. Their findings could shed light on the role of auto-antibodies in exacerbating COVID-19 severity.
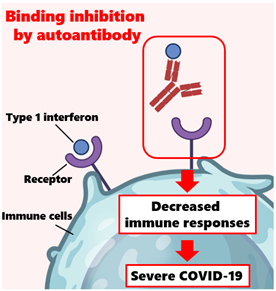
Image title: Auto-antibodies can neutralize type 1 interferons and compromise the immune response
Image caption: Auto-antibodies erroneously target their own proteins and enzymes. When these auto-antibodies target type 1 interferons, they can neutralize their function as signal proteins for the immune system. This study showed that some severe cases of COVID-19 were associated with the presence of auto-antibodies to type 1 interferons, shedding light on why some COVID-19 infections are severe without any other obvious risk factors.
Image credit: Chiaki Iwamura from Chiba University
Image license: Original content
Usage restrictions: Cannot be reused without permission
Even though COVID-19 manifests as a mild and short-lived disease in most people, some suffer extremely severe symptoms; in the worst cases, these patients die due to complications such as respiratory failure or thromboembolism. It is well-known that factors such as age and underlying medical conditions like diabetes or immunodeficiencies increase vulnerability to severe COVID-19. However, some patients still experience severe COVID-19 without any apparent reason.
One possible explanation may lie in auto-antibodies, which are antibodies that erroneously target specific proteins produced by one’s own body. In normal circumstances, type I interferons (or ‘t1-IFNs’) play a crucial role in the body’s defense against viral infections; they interfere with viral replication and help mobilize the immune system. However, auto-antibodies against t1-IFNs can neutralize their activity, compromising the body’s defense mechanisms. While detecting these auto-antibodies was uncommon before COVID-19, there have been multiple reports of severe COVID-19 patients bearing them since the pandemic started. Could auto-antibodies targeting t1-IFNs be more common than previously thought?
To answer this question, a research team, including Lecturer Chiaki Iwamura from Chiba University, Japan, investigated whether and how auto-antibodies targeting t1-IFNs are related to COVID-19 severity by analyzing blood samples from 123 Japanese patients. Their findings were published in Volume 44 of the Journal of Clinical Immunology on April 22, 2024. This research was co-authored by Dr. Kiyoshi Hirahara and Dr. Koutaro Yokote from Chiba University, as well as Dr. Ami Aoki from Niigata University.
The researchers first conducted an enzyme immunoassay to detect auto-antibodies to t1-IFNs in the blood samples, and then confirmed whether these antibodies could effectively neutralize t1-IFNs in cell cultures. “We found that three out of 19 severe and four out of 42 critical COVID-19 patients had neutralizing auto-antibodies to t1-IFNs. Interestingly, there were no characteristic clinical features among patients with auto-antibodies to t1-IFNs,” comments Dr. Iwamura. In other words, there were no pointers in the data as to why some COVID-19 patients developed these auto-antibodies, even when considering previous infections, treatments received, and underlying immune disorders. “Based on these findings, it is difficult to estimate the presence of auto-antibodies to t1-IFNs from the usual blood tests and clinical background,” remarks Dr. Iwamura.
To shed some light on how auto-antibodies to t1-IFNs affected COVID-19 patients, the researchers then conducted RNA sequencing and B cell receptor analyses. These experiments showed that conventional dendritic cells and canonical monocytes, two types of white blood cells, exhibited attenuated IFN signaling for patients in which auto-antibodies were present. Moreover, B cells (yet another type of immune cell) in these patients had fewer SARS-CoV-2-specific receptors, implying reduced effectiveness in combating an infection.
Overall, these findings highlight the importance of looking at auto-antibodies to t1-IFNs in more detail when facing viral epidemics. “People with auto-antibodies to t1-IFNs are more susceptible not only to SARS-CoV-2 but also to common viruses such as influenza and to unknown viruses that may emerge in the future,” warns Dr. Iwamura, “Thus, we hope to collaborate with companies to develop a system to detect auto-antibodies to t1-IFNs in the blood. Ideally, we would develop a test to examine the presence of these auto-antibodies in regular health checkups so that people will be able to know whether they have them with little burden.”
Let us hope their vision becomes a reality soon so that we can be better prepared to diagnose, prevent, and fight viral infections.
About Lecturer Chiaki Iwamura
Dr. Chiaki Iwamura obtained a PhD from Chiba University in 2008, where he served as Assistant Professor from 2008 to 2012. He was a Fellow of the National Institutes of Health, and is currently a Senior Lecturer at Chiba University. His research focuses on immunology, infectious disease, and allergies. He has published over 45 scientific papers in these fields. He is also a member of the Japan Society for Immunology.
Funding:
This work was supported by the following grants: Ministry of Education, Culture, Sports, Science and Technology (MEXT Japan) Grants-in-Aid for Scientific Research (Nos. JP19H05650, JP21H05120, JP21H05121, JP22H02885, JP22K15484, JP22K15485, JP23H02916); Transformative Research Areas (B) JP21H05120, and JP21H05121; Practical Research Project for Allergic Diseases and Immunology (Research on Allergic Diseases and Immunology) from the Japan Agency for Medical Research and Development, AMED (Nos. JP21ek0410060, JP21ek0410082 and JP19ek0410045); AMED-PRIME, AMED (Nos. JP20gm6110005, JP23ek0410092); AMED-CREST, AMED (Nos. JP21gm1210003, JP23gm1210003); AMED-SENTAN project (JP19hm0102069h001); JST FOREST Project (No. JPMJFR200R, Japan); Mochida Memorial Foundation for Medical and Pharmaceutical Research, MSD Life Science Foundation, Japanese Respiratory Foundation, the Japanese Association for Infectious Diseases, Grant for Clinical Research Promotion and Takeda Science Foundation.
Reference:
Title of original paper: Suppression of Type I Interferon Signaling in Myeloid Cells by Autoantibodies in Severe COVID-19 Patients
Authors: Ami Aoki1,2, Chiaki Iwamura1,3, Masahiro Kiuchi1, Kaori Tsuji1, Atsushi Sasaki1, Takahisa Hishiya1, Rui Hirasawa1, Kota Kokubo1, Sachiko Kuriyama1, Atsushi Onodera1, Tadanaga Shimada4, Tetsutaro Nagaoka5, Satoru Ishikawa6, Akira Kojima6, Haruki Mito7, Ryota Hase7, Yasunori Kasahara8, Naohide Kuriyama9, Sukeyuki Nakamura10, Takashi Urushibara11, Satoru Kaneda12, Seiichiro Sakao13, Osamu Nishida9, Kazuhisa Takahashi5, Motoko Y. Kimura3,14, Shinichiro Motohashi15, Hidetoshi Igari16,17, Yuzuru Ikehara18, Hiroshi Nakajima3,17,19, Takuji Suzuki3,20, Hideki Hanaoka3,21, Taka‐aki Nakada4, Toshiaki Kikuchi2, Toshinori Nakayama1,22, Koutaro Yokote23, and Kiyoshi Hirahara1,3,22
Affiliations:
- Department of Immunology, Graduate School of Medicine, Chiba University
- Department of Respiratory Medicine and Infectious Diseases, Niigata University Graduate School of Medical and Dental Sciences
- Synergy Institute for Futuristic Mucosal Vaccine Research and Development, Chiba University
- Department of Emergency and Critical Care Medicine, Graduate School of Medicine, Chiba University
- Department of Respiratory Medicine, Juntendo University Faculty of Medicine and Graduate School of Medicine
- Funabashi Central Hospital
- Department of Infectious Diseases, Japanese Red Cross Narita Hospital
- Department of Respiratory Medicine, Eastern Chiba Medical Center
- Department of Anesthesiology and Critical Care Medicine, School of Medicine, Fujita Health University
- Funabashi Municipal Medical Center
- Kimitsu Chuo Hospital
- Department of Gastroenterology, NHO Chiba Medical Center
- Department of Pulmonary Medicine, International University of Health and Welfare Narita Hospital
- Department of Experimental Immunology, Graduate School of Medicine, Chiba University
- Department of Medical Immunology, Graduate School of Medicine, Chiba University
- Department of Infectious Diseases, Chiba University Hospital
- COVID-19 Vaccine Center, Chiba University Hospital
- Department of Pathology, Graduate School of Medicine, Chiba University
- Department of Allergy and Clinical Immunology, Graduate School of Medicine, Chiba University
- Department of Respirology, Graduate School of Medicine, Chiba University
- Clinical Research Center, Chiba University Hospital
- AMED-CREST, AMED
- Department of Endocrinology, Hematology and Gerontology, Graduate School of Medicine, Chiba University
Journal: Journal of Clinical Immunology
DOI: 10.1007/s10875-024-01708-7
Contact: Chiaki Iwamura
Graduate School of Medicine, Chiba University
Email: iwamurac@chiba-u.jp
Public Relations Office, Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: koho-press@chiba-u.jp
Tel: +81-43-290-2018
Recommend
-

Reassessing the role of “Forest Resources” in achieving carbon neutrality: Measuring forests with the combination of drones, mathematics, and computer graphics
2023.09.12
-

The Joy of Work and The Delight of Nurturing: From Social Farming to Universal Farms
2025.02.10
-

The reciprocity of life: The interactive relationships between people, plants, and the environment
2023.05.19


