Researchers have developed a novel one-step method to achieve selective bromination at the specific position of indole rings.
The development of drugs through chemical modifications of naturally occurring indole alkaloids has emerged as an attractive research area. However, due to their reactivity, the selective functionalization at the C5 position of the indole ring has been difficult. Recently, researchers developed a new one-step method for C5-selective bromination of indole alkaloids. It is simple, fast, mild, and can accommodate various functional groups. This innovative method can lead to the development of new drugs and agrochemicals.
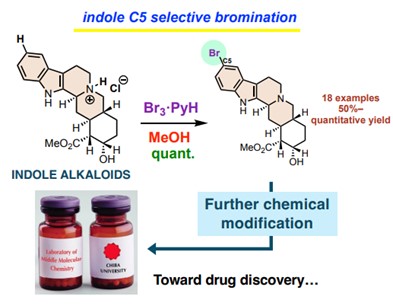
Image title: A Novel Method for Indole C5-Selective Bromination of Indole Alkaloids
Image caption: The proposed method is simple, mild, fast, and produces high yields, with the potential to inspire the development of new drugs and agrochemicals.
Image credit: Hayato Ishikawa from Chiba University
Image license: Original Content
Usage Restrictions: Cannot be reused without permission
Indolo[2,3-a]quinolizidine is a common structural motif in various natural products. Its molecular structure contains the indole ring, a functional group in tryptophan. Tryptophan, an essential amino acid, is required for the production and maintenance of our body’s proteins, muscles, enzymes, and neurotransmitters. Many natural products are derived from it. Over 3,000 monoterpene indole alkaloids (MTIAs), which are natural products consisting of indole rings, have been found in plants, and some of them have been used as medicines. For example, vinblastine has been used as an anticancer drug, and reserpine is used to treat high blood pressure.
The indole ring itself consists of a six-membered benzene ring fused with a five-membered nitrogen-containing pyrrole ring. Functionalization at the C5 position of the indole core of MTIAs is a common occurrence in biosynthesis and such MTIAs demonstrate attractive biological activities, including medicinal activity. Hence, the development of drugs through chemical modifications of the naturally occurring MTIAs as templates has attracted significant attention. Consequently, the exploration of chemical modification methods for the indole ring has become an important research area. However, the indole ring is highly reactive, and hence, selective chemical modifications at its C5 reaction site have been difficult.
Addressing this challenge, a team of researchers from the Graduate School of Pharmaceutical Sciences at Chiba University, including Go Yoshimura (first author), Jukiya Sakamoto, and Mariko Kitajima, led by Professor Hayato Ishikawa, developed a novel one-step process for indole C5-selective bromination of MTIAs. Prof. Ishikawa explains, “Bromine is an extremely important functional group due to its versatility. It serves as a substrate for key reactions and for facilitating further functionalization. We have successfully achieved selective bromination at the indole C5 position, which has been a notoriously challenging feat.”
Their study, published in Chemistry–A European Journal on April 7, 2024, was highly recommended at the time of the review owing to its importance and was also selected as the cover picture.
The researchers previously achieved selective C5-bromination of an indole alkaloid. However, it required a four-step process and was not suitable for other multi-functionalized substrates. In this study, the team developed a simpler one-step process. They used indolo[2,3-a]quinolizidine as a model substrate and treated it with equal amounts of pyridinium tribromide and hydrochloric acid with methanol as the solvent at 0 °C. The resulting reaction was surprisingly fast, requiring only 10 minutes, yielding the desired C5-brominated derivative. The researchers further found that an in-situ generated C3-, C5-brominated indolenine intermediate served as the brominating agent. They separately synthesized this intermediate and experimentally verified its role.
The proposed innovative method is simple, operates under mild reaction conditions, accommodates various functional groups, and produces high yields. Furthermore, this method does not require any special protection or directing groups. The researchers demonstrated this method’s versatility by preparing various brominated indole alkaloids, including a brominated product prepared from naturally occurring yohimbine.
Highlighting the importance of the study, Prof. Ishikawa says, “The chemical modification method proposed in this study will be beneficial for the development of new drugs and agrochemicals. It will also pave the way for the development of additional indole chemical reactions as scientists further explore the newfound reactivity of indoles.”
The team hopes that the development of new medicines and pesticides using their method will contribute to a healthier society.
About Professor Hayato Ishikawa
Hayato Ishikawa is currently a Professor at the Graduate School of Pharmaceutical Sciences, Chiba University, Japan. He received his Ph.D. from Chiba University in 2004. He also leads the Ishikawa group, which focuses on the total synthesis of biologically active natural products using originally developed new synthetic methods, isolation, and structure elucidation of natural products from botanical medicinal resources. He received the Pharmaceutical Society of Japan Award for Divisional Scientific Promotion in 2024 and the Nagase Science Foundation Award in 2021. He has over 100 publications with over 6,000 citations. His research interests include organic synthesis and total synthesis.
Funding:
We gratefully acknowledge financial support through a Grant-in-Aid for Scientific Research (B) (JP21H02608 to H. I.) and Grant-in-Aid for Challenging Research (Pioneering, JP23K18174 to H. I.) from JSPS, and a JSPS Research Fellowships for Young Scientists (JP21J20696) to J. S.
Reference:
Title of original paper: Indole C5-Selective Bromination of Indolo[2,3-a]quinolizidine Alkaloids via In Situ-Generated Indoline Intermediate
Authors: Go Yoshimura1, Jukiya Sakamoto1, Mariko Kitajima1, and Hayato Ishikawa1*
Affiliations: 1Graduate School of Pharmaceutical Sciences, Chiba University, Japan
Journal: Chemistry–A European Journal
DOI: 10.1002/chem.202401153
Contact: Professor Hayato Ishikawa
Graduate School of Pharmaceutical Sciences, Chiba University
Email: h_ishikawa@chiba-u.jp
Public Relations Office, Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: koho-press@chiba-u.jp
Tel: +81-43-290-2018
Recommend
-

Nurturing Teaching Personnel for Multicultural Societies: High Schools-Universities-Graduate Schools’ Tripartite Collaboration to Develop Educational Programs in ASEAN Countries
2023.08.25
-

The Science Behind Nature’s Touch: Horticultural Therapy and Community Well-Being
2025.01.14
-

Designing a Comfortable Living: A Kampo Clinic that Simulates the Five Senses
2024.01.26


