Researchers found that the G900 region in mice genome plays a crucial role in inflammatory pathways, especially after exposure to allergens
Type-2 helper T (Th2) cells play intricate roles in the inflammatory response underlying asthma. Elevated levels of the protein GATA3 stimulate the maturation of these cells. Consequently, gene regions known as enhancers, which enhance the expression of the GATA3 gene, have been implicated in asthma-associated inflammation. A study has revealed that the enhancer region G900 may be pivotal in the inflammatory response mediated by Th2 cells, offering clues for new therapeutic strategies.
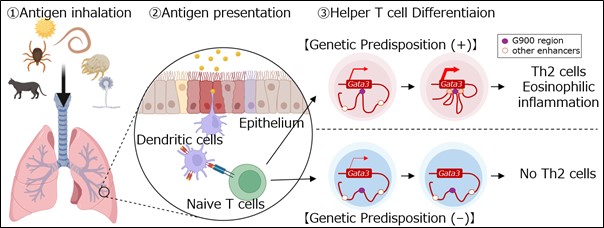
Image title: The G900 region induces in vivo Th2 cell differentiation through the optimization of chromatin structure.
Image caption: Researchers identify G900-dependent mechanism of in vivo Th2 differentiation.
Image credit: Arifumi Iwata from Chiba University
Image license: Original Content
Usage restrictions: Cannot be reused without permission
Asthma patients experience respiratory distress due to allergens like house dust mites or pollen. However, the various triggers for asthma share a common pathway involving the release of proteins called type-2 cytokines by Type-2 helper T (Th2) cells and group-2 innate lymphoid cells (ILC2s). Both Th2 and ILC2 require high amounts of GATA-binding protein 3 (GATA3) for their maturation.
Specific gene sequences called enhancers are responsible for elevating the expression of GATA3 genes in humans. Studies have found that by controlling the production of GATA3, enhancers influence the development of Th2 and ILC2. The gene region G900, located close to the GATA3 gene, is currently being investigated for its role in the asthma inflammation pathway. In a recent breakthrough, a study by researchers from Chiba University that will be published in Proceedings of the National Academy of Sciences, USA, on the week of June 24, 2024, has discovered that the mouse gene region corresponding to the human G900 is involved in Th2 differentiation and consequently in enhancing allergic responses, although not ILC2.
“Multiple genome-wide association studies (GWAS) have aimed to elucidate the underlying biology and predict susceptibility to asthma. The importance of single nucleotide polymorphisms (SNPs) within the 10p14 locus has been indicated in several independent studies, not only in asthma susceptibility but also in a broad spectrum of allergic diseases, including allergic rhinitis, atopic dermatitis, and eosinophilic granulomatosis with polyangiitis”, explains Professor Hiroshi Nakajima of Chiba University, the lead researcher in the study.
To investigate the role of G900 in asthma-associated inflammatory pathways, researchers including Arifumi Iwata, Takashi Kumagai, and Hiroki Furuya of the Graduate School of Medicine at Chiba University, among others, developed mice that lacked the G900 region in their genome. These mG900 “knockout mice” were subsequently exposed to allergens such as papain and house dust mites. Researchers discovered that mG900 knockout mice produce reduced inflammatory response compared to control mice with intact mG900 when exposed to house dust mites. Further, they also found suppression of Th2 differentiation in mG900 knockout mice compared to control mice.
“One aspect we elucidate in our study is the role of the murine G900 region in Th2 differentiation and allergic airway inflammation”, Prof. Nakajima explains. “This region, homologous to the human G900 region associated with asthma, is shown to be essential for in vivo Th2 cell differentiation and allergic responses, particularly in the context of house dust mite (HDM)-induced allergic airway inflammation. Furthermore, we have demonstrated that this G900 region is crucial for optimizing the three-dimensional chromatin structure near GATA3 in Th2 cells”, he further states.
The study’s findings have wide-ranging implications for asthma care as well as therapeutic interventions for other allergic diseases. In the future, methods of regulating Th2 differentiation and function by pharmacologically restricting GATA3 enhancers like G900 may help reduce exaggerated immune responses underlying allergic reactions.
“By identifying and understanding critical genetic regions that regulate immune responses, such as the mG900 region, it may be possible to develop precision medicine approaches tailored to individual genetic profiles. This could lead to more effective and personalized treatments, reducing the incidence and severity of allergic reactions and improving the quality of life for individuals suffering from these conditions”, Prof. Nakajima concludes.
About Professor Hiroshi Nakajima of Chiba University
Hiroshi Nakajima is currently a Professor at the Chiba University’s Graduate School of Medicine. Prof. Nakajima has published nearly 250 scientific articles on the molecular underpinnings of refractory allergic or autoimmune diseases. His research bridges basic research and clinical medicine by helping expedite the development of new medicines for allergies and autoimmune diseases.
Funding:
This study was supported in part by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government (MEXT), AMED (Grant Number 19ek0410043h0003), JST (Moonshot R&D) (Grant Number JPMJMS2025), Takeda Science Foundation, Japan, and The Basic Research Support Program from Japanese Society of Allergology 2020.
Reference:
Title of original paper: A distal enhancer of GATA3 regulates Th2 differentiation and allergic inflammation
Authors: Takashi Kumagai1, Arifumi Iwata1, Hiroki Furuya1, Kodai Kato1, Atsushi Okabe2,3, Yosuke Toda1, Mizuki Kanai1, Lisa Fujimura4, Akemi Sakamoto4,5, Takahiro Kageyama1, Shigeru Tanaka1, Akira Suto1, Masahiko Hatano4,5, Atsushi Kaneda2,3, and Hiroshi Nakajima1,6
Affiliations:
- Department of Allergy and Clinical Immunology, Graduate School of Medicine, Chiba University, Chiba, Japan
- Department of Molecular Oncology, Graduate School of Medicine, Chiba University, Chiba, Japan
- Health and Disease Omics Center, Chiba University
- Biomedical Research Center, Chiba University, Chiba, Japan
- Department of Biomedical Science, Graduate School of Medicine, Chiba University, Chiba, Japan
- Chiba University Synergy Institute for Futuristic Mucosal Vaccine Research and Development (cSIMVa), Chiba, Japan
DOI: https://doi.org/10.1073/pnas.2320727121
Contact: Hiroshi Nakajima
Graduate School of Medicine, Chiba University
Email: nakajimh@faculty.chiba-u.jp
Public Relations Office, Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: koho-press@chiba-u.jp
Tel: +81-43-290-2018





