Researchers identify novel transcriptional enhancers regulating gene expression during neuronal differentiation
Genome-wide association studies (GWASs) have linked genetic variants to neuropsychiatric disorders, but their regulatory roles in non-coding regions remain largely unclear. Using the LUHMES neuronal cell model, researchers identified and characterized thousands of enhancers active during neuronal differentiation, linked them to target genes, and validated key interactions. This study demonstrates a significant enrichment of GWAS variants associated with Parkinson’s disease and schizophrenia within these enhancers, providing a valuable resource for understanding neuronal development.
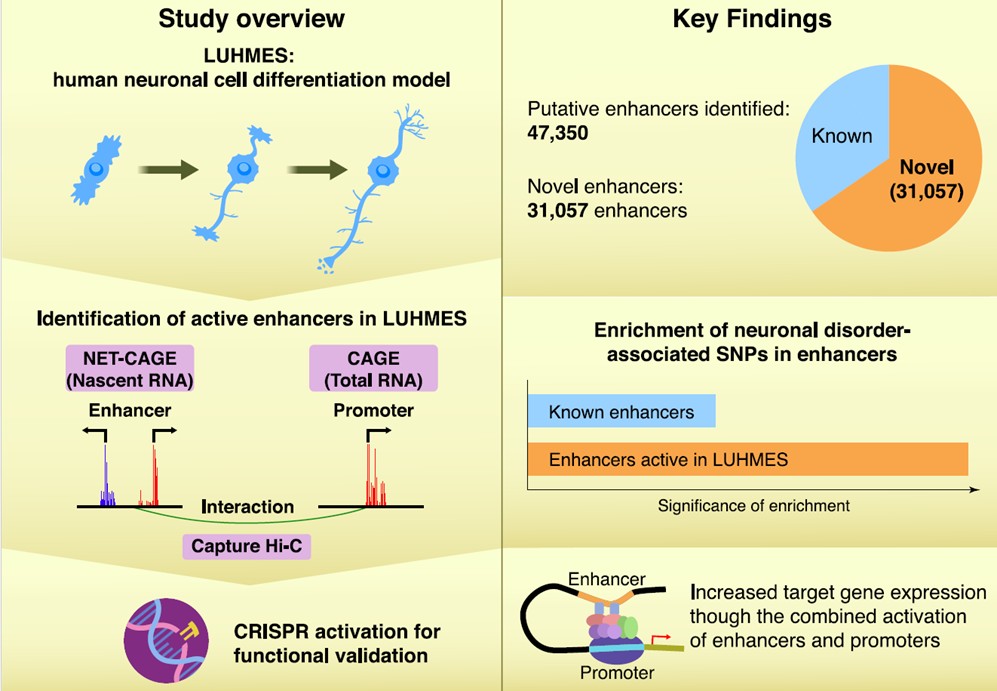
Image title: Identification of novel enhancers regulating gene expression in neuronal differentiation and neuropsychiatric disorders
Image caption: New study by Chiba University, Japan in collaboration with Karolinska Institutet, Sweden, uncovers novel enhancer-promoter interactions that regulate gene expression during neuronal differentiation and neurological disorders, paving the way for the identification of novel druggable targets.
Image credit: Associate Professor Masahito Yoshihara, Chiba University
Image license: Original content
Usage Restrictions: Cannot be used without permission
Neuropsychiatric disorders are becoming increasingly prevalent. Given their complex and multifactorial pathogenesis, there is an urgent need for effective and targeted therapies that can improve patients’ quality of life. Genome-wide association studies (GWASs) have identified various genetic alterations that contribute to the development and progression of neuropsychiatric disorders, ranging from mild dyslexia to more severe conditions such as schizophrenia.
While thousands of single nucleotide polymorphisms (SNPs)—changes in a single nucleotide position in the DNA—have been associated with neurological disorders, the majority are located in non-coding regions of the genome. Although these non-coding regions do not encode proteins, they contain regulatory elements such as enhancer sequences, which play crucial roles in controlling gene expression. Enhancers can regulate gene activity over long distances and are often specific to cell types and developmental stages. Despite their importance, enhancers remain poorly characterized, and their precise functions in neurological development and disease are not yet fully understood.
In a new study, Associate Professor Masahito Yoshihara from the Institute for Advanced Academic Research and Graduate School of Medicine at Chiba University, along with Professor Juha Kere and Dr. Peter Swoboda from the Department of Medicine Huddinge (MedH) at Karolinska Institutet, Sweden, and Dr. Pelin Sahlén from KTH – Royal Institute of Technology, Sweden, aimed to bridge this knowledge gap. They conducted a series of advanced analyses to identify and characterize enhancers involved in neuronal differentiation using LUHMES cells, a cell line derived from human fetal mesencephalic dopaminergic neurons.
Giving further insight into their work, soon to be published in EMBO Reports, Dr. Yoshihara, the main author of the study, says, “Elucidating how disease-associated variants influence gene regulation can uncover previously unidentified molecular pathways involved in neuronal disorders and reveal novel therapeutic targets for drug development.”
The researchers used LUHMES neuronal precursor cells, which can differentiate into functional neurons with a high transcriptional similarity to human brain-derived neurons. They employed Cap Analysis of Gene Expression (CAGE) and Native Elongating Transcript (NET)-CAGE to identify and quantify the activity of promoters and enhancers at a genome-wide level. These techniques were combined with targeted chromosome conformation capture (Capture Hi-C/HiCap), an advanced sequencing method that links distant enhancers to their target genes. They also examined the associations between putative enhancers and GWAS-identified loci implicated in neuronal disorders. The analysis identified 47,350 active putative enhancers, 65.6% of which were novel, and demonstrated an enrichment of SNPs associated with Parkinson’s disease, schizophrenia, bipolar disorder, and major depressive disorder within these enhancers.
Finally, they conducted in vitro assays in cultured cells to functionally validate promoter-enhancer interactions. Using the CRISPR-Cas9 system for genome editing, they activated enhancers and promoters of genes involved in neuronal differentiation and disorders. Consistent with their analysis, enhancer activation led to significant increases in the expression levels of the target genes.
Overall, these findings shed light on novel enhancer-promoter interactions and variants located within enhancer sequences, with potential pathogenic implications. These interactions and their target genes can further uncover novel druggable targets for the development of therapies against debilitating neuronal disorders.
Highlighting the clinical applications of their work, Dr. Yoshihara says, “Our study further exemplifies the power of enhancer discovery in providing potential clues to better understand the pathogenesis of neuropsychiatric disorders. Our results highlight the vast regulatory potential embedded in non-coding regions that harbor relevant enhancers and provide a valuable resource for future studies on neuronal development, regulation, and disorders.”
This study represents an important step toward uncovering novel gene regulatory mechanisms involved in neuronal development and disease, paving the way for the discovery of new therapeutic strategies for debilitating neuropsychiatric conditions.
About Associate Professor Masahito Yoshihara
Dr. Masahito Yoshihara, Associate Professor at the Institute for Advanced Academic Research and the Graduate School of Medicine, Chiba University, focuses on identifying diagnostic markers and therapeutic targets through multi-omics integration, including transcriptome and proteome analyses from patient samples. He also investigates gene regulatory mechanisms during cellular differentiation using advanced in vitro models derived from stem and progenitor cells. His research aims to enhance our understanding of disease pathogenesis while improving methodologies for stem cell reprogramming and differentiation. These efforts pave the way for innovative approaches in regenerative medicine and the development of targeted therapies.
Funding:
This work was supported by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) grant number 22K20622, Carl Tryggers Stiftelse (CTS) grant agreement No CTS-19311, Swedish Research Council, Swedish Brain Foundation, Sigrid Jusélius Foundation, Jane and Aatos Erkko Foundation, KI Strategic Neuroscience Program, Torsten Söderberg Foundation, Olle Engkvist Foundation, Åhlén Foundation, Karolinska Institute PhD student (KID) Fellowship, JSPS Postdoctoral Fellowship for Research in Japan, and Chinese Scholarship Council (CSC) PhD student Fellowship.
Reference:
Title of original paper: Transcriptional enhancers in human neuronal differentiation provide clues to neuronal disorders
Authors: Masahito Yoshihara1,2,3,4, Andrea Coschiera1, Jorg A. Bachmann5, Mariangela Pucci1,6, Haonan Li1, Shruti Bhagat7, Yasuhiro Murakawa7,8,9,10, Jere Weltner11,12,
Eeva-Mari Jouhilahti11,12, Peter Swoboda1, Pelin Sahlen5, Juha Kere1,11,12
Affiliations:
- Department of Medicine Huddinge (MedH), Biosciences and Nutrition Unit, Karolinska Institutet, Stockholm, Sweden
- Institute for Advanced Academic Research, Chiba University, Chiba, Japan
- Department of Artificial Intelligence Medicine, Graduate School of Medicine, Chiba University, Chiba, Japan
- remium Research Institute for Human Metaverse Medicine (WPI-PRIMe), Osaka University, Suita, Osaka, Japan
- Science for Life Laboratory, KTH – Royal Institute of Technology, Stockholm, Sweden
- Department of Bioscience and Technology for Food, Agriculture and Environment, University of Teramo, Teramo, Italy
- Institute for the Advanced Study of Human Biology, Kyoto University, Kyoto, Japan
- RIKEN-IFOM Joint Laboratory for Cancer Genomics, RIKEN Center for Integrative Medical Sciences, Yokohama, Japan
- IFOM – the FIRC Institute of Molecular Oncology, Milan, Italy
- Department of Medical Systems Genomics, Graduate School of Medicine, Kyoto University, Kyoto, Japan
- Folkhalsan Research Centre, Helsinki, Finland
- Stem Cells and Metabolism Research Program, University of Helsinki, Finland
Journal: EMBO Reports
DOI: 10.1038/s44319-025-00372-1
Contact:
Masahito Yoshihara
Institute for Advanced Academic Research and Graduate School of Medicine, Chiba University
Email: masahito.yoshihara@chiba-u.jp
Public Relations Office, Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: koho-press@chiba-u.jp
Tel: +81-43-290-2018





