A novel radioactive drug developed by researchers demonstrates potential for targeting and treating metastatic melanoma
Metastatic melanoma is the most aggressive form of skin cancer. In an effort to achieve targeted therapy for metastatic melanoma, researchers from Japan recently developed a new radioactive drug that emits alpha particles. With their short range of emission and high energy, alpha particles are particularly promising for targeted therapies that require high energy release in the cancer tissue while minimizing the damage to nearby healthy tissues.
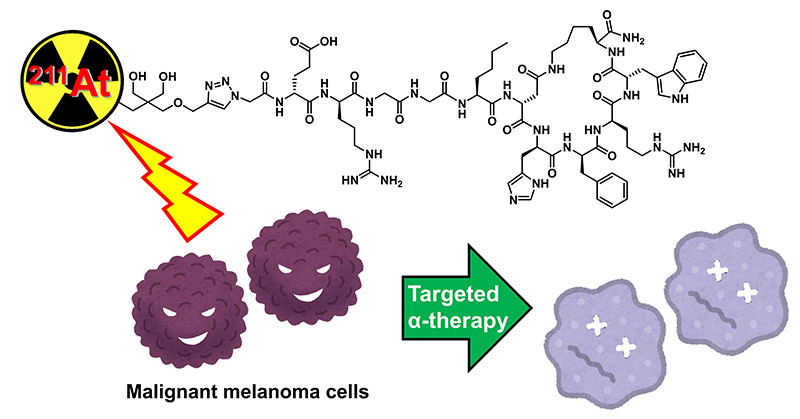
Image title: Targeted alpha therapy as a new therapeutic strategy for treating metastatic melanoma.
Image caption: A newly developed astatine-211 (211At)-labeled peptide drug inhibits malignant melanoma cells by releasing alpha particles in the targeted cancer tissue.
Image credit: Dr. Hiroyuki Suzuki from Chiba University, Japan
Image license: Original content
Usage restrictions: Cannot be used without permission.
Metastatic melanoma, also known as stage IV melanoma, is a type of skin cancer that spreads to other parts of the body. It is one of the most aggressive forms of skin cancer, with current therapies—including immunotherapy and targeted drugs—showing limited effectiveness. Radiotherapy is an emerging treatment for melanoma, but conventional beta-emitting radionuclide therapies have limitations due to their low energy transfer and long-range radiation, which can cause unintended damage to healthy tissues.
To enhance the efficacy of radiotherapy, a research team from Japan, led by Assistant Professor Hiroyuki Suzuki from Chiba University, including Dr. Tomoya Uehara from Chiba University, Dr. Noriko S. Ishioka from National Institutes for Quantum Science and Technology, Dr. Hiroshi Tanaka from Juntendo University, Dr. Tadashi Watabe from Osaka University, adopted targeted alpha therapy (TAT) as a promising alternative to conventional beta therapy. They developed an astatine-211 (211At)-labeled peptide drug that could offer a potential breakthrough for treating metastatic melanoma. The research was conducted in collaboration with the National Institutes for Quantum Science and Technology and was published in the European Journal of Nuclear Medicine and Molecular Imaging on January 20, 2025.
TAT is a form of radiotherapy that involves drugs labeled with alpha particle-emitting radioisotopes. Compared to other forms of radioactive emissions (beta and gamma emissions), alpha particles are heavier and therefore have a short range. Owing to their greater mass, alpha particles also carry relatively higher energy, which is beneficial for the disruption of cancer cells.
To develop the treatment, the researchers first identified an optimal hydrophilic linker to enhance tumor targeting and reduce off-target accumulation. The team then designed an astatine-211(211At)-labeled α-melanocyte-stimulating hormone (α-MSH) peptide analog called [211At]NpG-GGN4c to specifically target melanocortin-1 receptors (MC1R), which are overexpressed in melanoma cells. “Since the tagged peptide was also receptor-targeted, it allowed for a high tumor selectivity while minimizing radiation exposure to the surrounding tissues,” comments Dr. Suzuki.
The synthesized peptides were then tested on B16F10 melanoma-bearing mice models, following which they conducted a biodistribution analysis where the team compared tumor uptake, clearance from organs, and the overall stability of the compound. Dr. Uehara elaborates on the methodology, saying, “We treated the mice with different doses of the compound while monitoring the tumor response, body weight, and survival rates over time. We found a dose-dependent inhibitory effect in a melanoma-bearing mouse model, confirming the effectiveness of our approach.”
The findings were remarkable. The [211At]NpG-GGN4c showed high accumulation in tumors and rapid clearance from non-target organs, confirming its specificity for MC1R on melanoma cells. Monitoring tumor growth revealed significant tumor suppression in a dose-dependent manner. Furthermore, [211At]NpG-GGN4c also demonstrated high stability in blood plasma, minimizing the risk of radioactive leakage in the body.
Hailing the exciting results, Dr. Suzuki affirms that the molecular design of their synthesized drug could be useful for developing other 211At-labeled radiopharmaceuticals. He says, “We believe our approach could open up new possibilities for treating refractory cancers beyond melanoma.”
The team is also hopeful about promoting a clinical application of 211At-based TAT. “If successfully translated into human trials, this therapy may emerge as a viable treatment option for patients with advanced melanoma in the coming years,” speculates Dr. Suzuki. “This could provide new therapeutic opportunities for patients with refractory cancer.”
About Assistant Professor Hiroyuki Suzuki from Chiba University
Dr. Hiroyuki Suzuki is an Assistant Professor at the Graduate School of Pharmaceutical Sciences, Chiba University. He earned his doctoral degree from Chiba University in 2013. His research focuses on the development of radiopharmaceuticals. He has made significant contributions to the scientific community through his groundbreaking research focused on radiotheranostics. In November 2024, Dr. Suzuki received the Best Young Investigator Award from the Japanese Society of Nuclear Medicine in recognition of his outstanding contributions to the field.
Funding:
This work was supported by a Grant-in-Aid for Scientific Research (C) (22K07686), a Fund for the Promotion of Joint International Research (Fostering Joint International Research) (23KK0291) from the Japan Society for the Promotion of Science (JSPS), and JSPS Grant-in-Aid for Scientific Research on Innovative Areas, grant number 22H04924.
Reference:
Title of original paper: An 211At-labeled alpha-melanocyte stimulating hormone peptide analog for targeted alpha therapy of metastatic melanoma
Authors: Hiroyuki Suzuki1, Saki Yamashita1, Shoko Tanaka1, Kento Kannaka1, Ichiro Sasaki2, Yasuhiro Ohshima,2, Shigeki Watanabe2, Kazuhiro Ooe3, Tadashi Watabe3,4, Noriko S. Ishioka2, Hiroshi Tanaka5,6, and Tomoya Uehara1
Affiliations:
- Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1 Inohana, Chuo-ku, Chiba 260-8675, Japan.
- Department of Quantum-Applied Biosciences, Takasaki Institute for Advanced Quantum Science, National Institutes for Quantum Science and Technology, 1233 Watanuki, Takasaki, Gunma 370-1292, Japan.
- Institute for Radiation Sciences, Osaka University, 1-1 Machikaneyama, Toyonaka, Osaka 560-0043, Japan.
- Department of Radiology, Graduate School of Medicine, Osaka University, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
- Faculty of Pharmacy, Juntendo University, 6-8-1 Hinode, Urayasu, Chiba, 279-0013 Japan.
- Department of Chemical Science and Engineering, Institute of Science Tokyo, 2-12-1 Ookayama, Meguro-ku, Tokyo 152-8552, Japan.
Journal: European Journal of Nuclear Medicine and Molecular Imaging
DOI: 10.1007/s00259-024-07056-3
Contact: Hiroyuki Suzuki
Assistant Professor at the Graduate School of Pharmaceutical Sciences, Chiba University
Email: h.suzuki@chiba-u.jp
Public Relations Office, Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: koho-press@chiba-u.jp
Tel: +81-43-290-2018
National Institutes for Quantum Science and Technology
Address: 4-9-1 Anagawa, Inage-ku, Chiba-shi, Chiba 263-8555 JAPAN
Email: global@qst.go.jp
Tel: +81-43-3025
Recommend
-

Taking up Immunological Research against Incurable Diseases−The Significance of “1” Life
2023.01.12
-

From ELSI to RRI: Exploring the Ethical Landscape of Science and Technology
2024.11.21
-

Towards a self-sustainable natural environment: The science of restoration ecology, where researchers engage in conversation with the earth
2023.02.02


