Researchers characterize leaf lipid droplet proteins, shedding light on their potential roles in lipid movement and fat production catalysis
Lipid droplets (LDs) serve as storage organelles found in plant leaves and seeds. While the functions of seed LD proteins in lipid metabolism are well understood, many leaf LD proteins remain unidentified. Scientists have now identified leaf LD proteins and uncovered their localization within Arabidopsis thaliana, shedding light on their potential roles in lipid metabolism and fat production. Their observations reveal that these proteins are associated with LD movement and lipid metabolism.
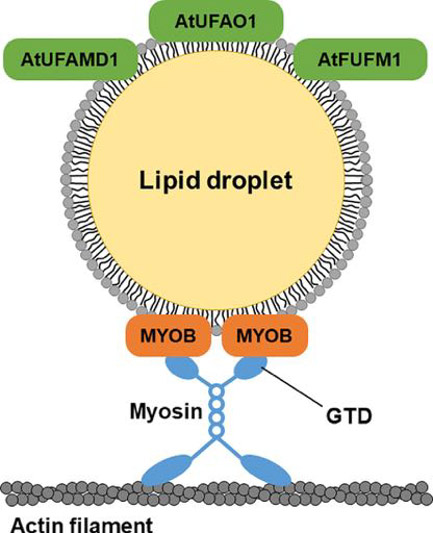
Image title: A schematic representation of multiple unique plant proteins localized in lipid droplets on the leaves.
Image caption: A new study shows that lipid droplets in the leaves contain plant proteins such as different myosin binding proteins involved in intracellular movement, and several enzymes responsible for furan-containing fatty acid biosynthesis—a type of fatty acid production in plants—highlighting previously undiscovered potential molecular functions.
Image credit: Takashi L. Shimada from Chiba University
Image Source link: https://www.frontiersin.org/files/Articles/1331479/fpls-15-1331479-HTML-r1/image_m/fpls-15-1331479-g006.jpg
Image license: CC BY 4.0
Lipids are biomolecules essential for the proper functioning of the living cell, ranging from comprising cell membranes to forming integral components of cell signaling pathways. Plant cells possess cell subcellular structures or organelles called lipid droplets (LDs) in the leaves and seeds, which store excess lipids (fats).
Recent studies have shown that LDs also localize unique plant proteins that perform essential molecular functions. For example, seed LDs localize plant proteins called oleosins, which help seeds weather freezing temperatures and germinate properly. With an expanding body of proof that LDs are not just organelles for carbon storage but may also perform essential molecular functions, a group of researchers from Japan sought to understand the role of the relatively unexplored leaf LDs. Associate Professor Takashi L. Shimada, an LD expert affiliated with Chiba University, explains the motivation behind their research, “We have a limited understanding of the functions of leaf LDs—why do plant leaves accumulate LDs at all? We believe the answer to this fundamental question would also contribute to lipid production technology.”
In a new pioneering study published in Frontiers in Plant Science on 01 March 2024, Dr. Shimada and his research group showed that leaf LDs localized myosin binding proteins essential for intracellular movements and enzymes that catalyze furan fatty acid biosynthesis—a type of fat production in living cells—highlighting the potential and previously undiscovered molecular functions of LDs. This research was led by Dr. Shimada, who is associated with the Graduate School of Horticulture, Plant Molecular Science Center, and Research Center for Space Agriculture and Horticulture at Chiba University, Japan. His research group also involved Mr. Yuto Omata from the Faculty of Horticulture, Chiba University, who is the first author of this study, along with Dr. Emi Mishiro-Sato, lecturer at World Premier International Research Center Initiative-Institute of Transformative Bio-Molecules, Nagoya University, and Dr. Ikuko Hara-Nishimura and Dr. Haruko Ueda from the Faculty of Science and Engineering, Konan University in Japan.
The researchers used an Arabidopsis plant model mutated to accumulate LDs in the leaves called Arabidopsis thaliana mutant high sterol ester 1. Dr. Shimada adds, “This Arabidopsis plant mutant model also serves as a valuable resource for further studying the leaf LD proteome.”
Further, the researchers isolated these leaf LDs and used mass spectrometry to identify associated proteins. They found 3,206 candidate proteins. Furthermore, they focused on 31 candidate proteins to test associated behavior in leaf LDs. They attached a fluorescent marker to these proteins to facilitate microscopic observations.
The fluorescence microscopy revealed the presence of MYOSIN BINDING PROTEIN14 (MYOB14) and two uncharacterized proteins in the leaf LDs under investigation. Further examination narrowed down the MYOB family members localized to leaf LDs: MYOB1, MYOB2, MYOB3, and MYOB5. The researchers also identified the two uncharacterized proteins as resembling enzymes for furan fatty acid biosynthesis, suggesting a potential link between leaf LDs and the type of fatty acid production.
Despite the progress made in this study, Dr. Shimada cautions about the need for further studies on leaf LDs, “We need to identify additional leaf LD proteins to resolve the molecular and physiological functions of leaf LDs.”
Nonetheless, the study paves the way for future research in leaf LDs—a relatively untapped plant resource—that could revolutionize lipid production and associated industries, all thanks to the efforts of Dr. Shimada and his team.
About Associate Professor Takashi L. Shimada
Dr. Takashi L. Shimada works in the Graduate School of Horticulture, Plant Molecular Science Center, and Research Center for Space Agriculture and Horticulture at Chiba University, Japan. Dr. Shimada investigates the intracellular structure of plants, especially lipid droplets. His research aims to comprehensively understand lipid storage in plants and enhance plant lipid production for potential application as a food resource. He has co-authored more than 20 reputed publications and is a member of multiple academic societies, including the Japanese Association of Plant Lipid Researchers and The Phytopathological Society of Japan.
Funding:
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) to TS (nos. 19K05809 and 23K17986), HU (nos. 19K06732 and 18H05496), and IH-N (no. 15H05776); Kato Memorial Bioscience Foundation (to TS); a SUNBOR GRANT from the Suntory Foundation for Life Sciences (to TS); Takeda Science Foundation (to TS); a research grant from The Yanmar Environmental Sustainability Support Association to TS; Inamori Foundation (to TS); JGC-S Scholarship Foundation (to TS); the Leading Initiative for Excellent Young Researchers (LEADER) from the Ministry of Education, Culture, Sports, Science and Technology in Japan (MEXT) to TS (no. J16HJ00026); the Phytochemical Plant Molecular Science of Strategic Priority Research Promotion Program from Chiba University (to TS and KS); and the Hirao Taro Foundation of KONAN GAKUEN for Academic Research (to HU and IH-N).
Reference:
Title of original paper:
Lipid droplets in Arabidopsis thaliana leaves contain myosin-binding proteins and enzymes associated with furan-containing fatty acid biosynthesis
Authors: Yuto Omata1, Reina Sato1, Emi Mishiro-Sato2, Keiko Kano2, Haruko Ueda3, Ikuko Hara-Nishimura3, and Takashi L. Shimada1,4,5,6*
Affiliations:
- Faculty of Horticulture, Chiba University, Matsudo, Japan
- World Premier International Research Center Initiative-Institute of Transformative Bio-Molecules, Nagoya University, Nagoya, Japan
- Faculty of Science and Engineering, Konan University, Kobe, Japan
- Graduate School of Horticulture, Chiba University, Matsudo, Japan
- Plant Molecular Science Center, Chiba University, Chiba, Japan
- Research Center for Space Agriculture and Horticulture, Chiba University, Matsudo, Japan
Journal: Frontiers in Plant Science
DOI: 10.3389/fpls.2024.1331479
Contact: Takashi L. Shimada
Faculty of Horticulture, Chiba University
Email: tlshimada@chiba-u.jp
Public Relations Office, Chiba University
Address: 1-33 Yayoi, Inage, Chiba 263-8522 JAPAN
Email: koho-press@chiba-u.jp
Tel: +81-43-290-2018
Recommend
-

Predicting Future CO2 Absorption and Emissions in Terrestrial Ecosystems: International Geostationary Meteorological Satellite Network for Accurate Estimation
2023.07.04
-

The Enigma of Time Perception: The Psychological Time Warp of ‘Tachypsychia’
2024.04.12
-

On the Road to Establishing a Performing Arts Medicine Center: Specialized Doctors for Musicians and Ballet Dancers
2023.06.02


